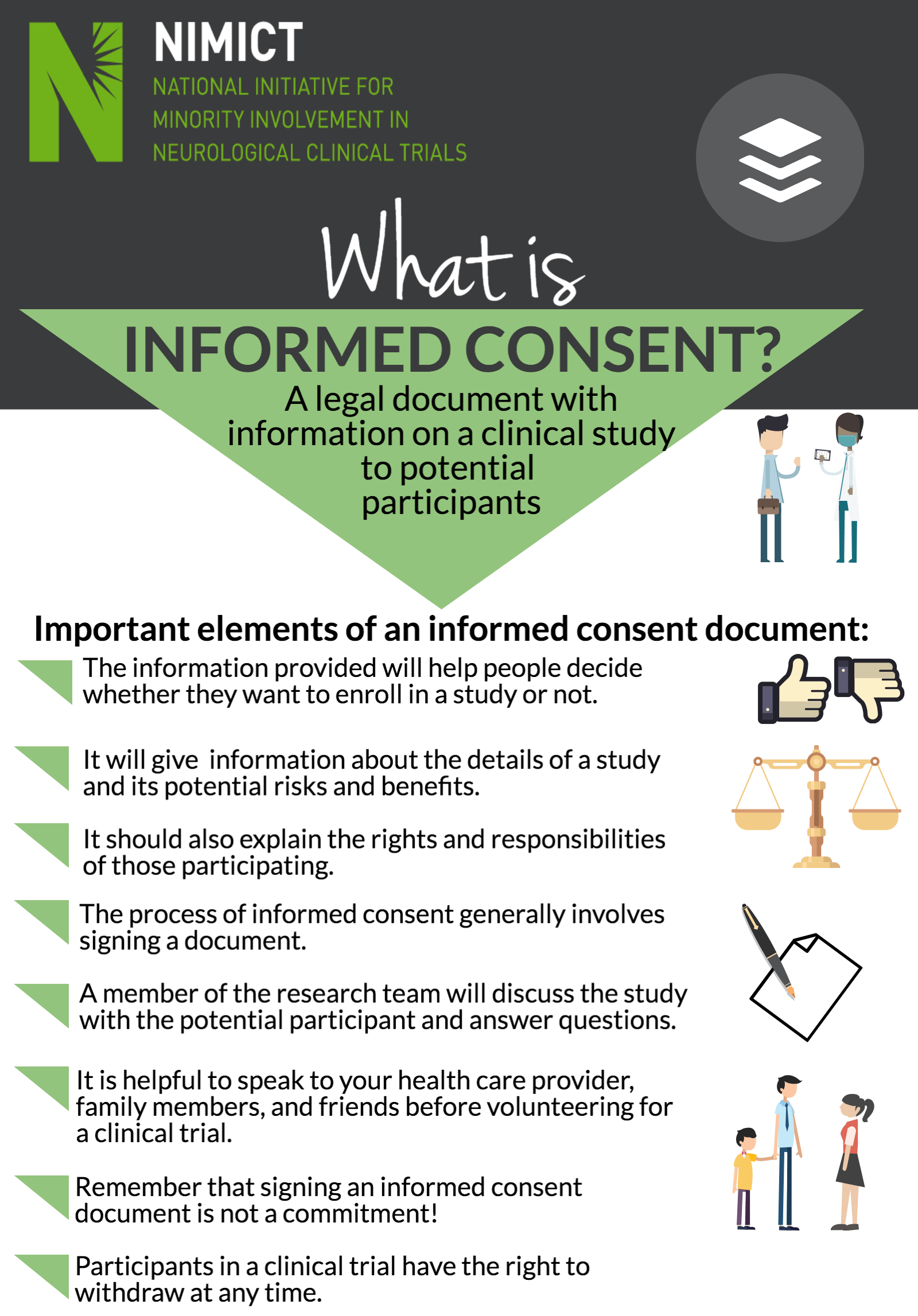
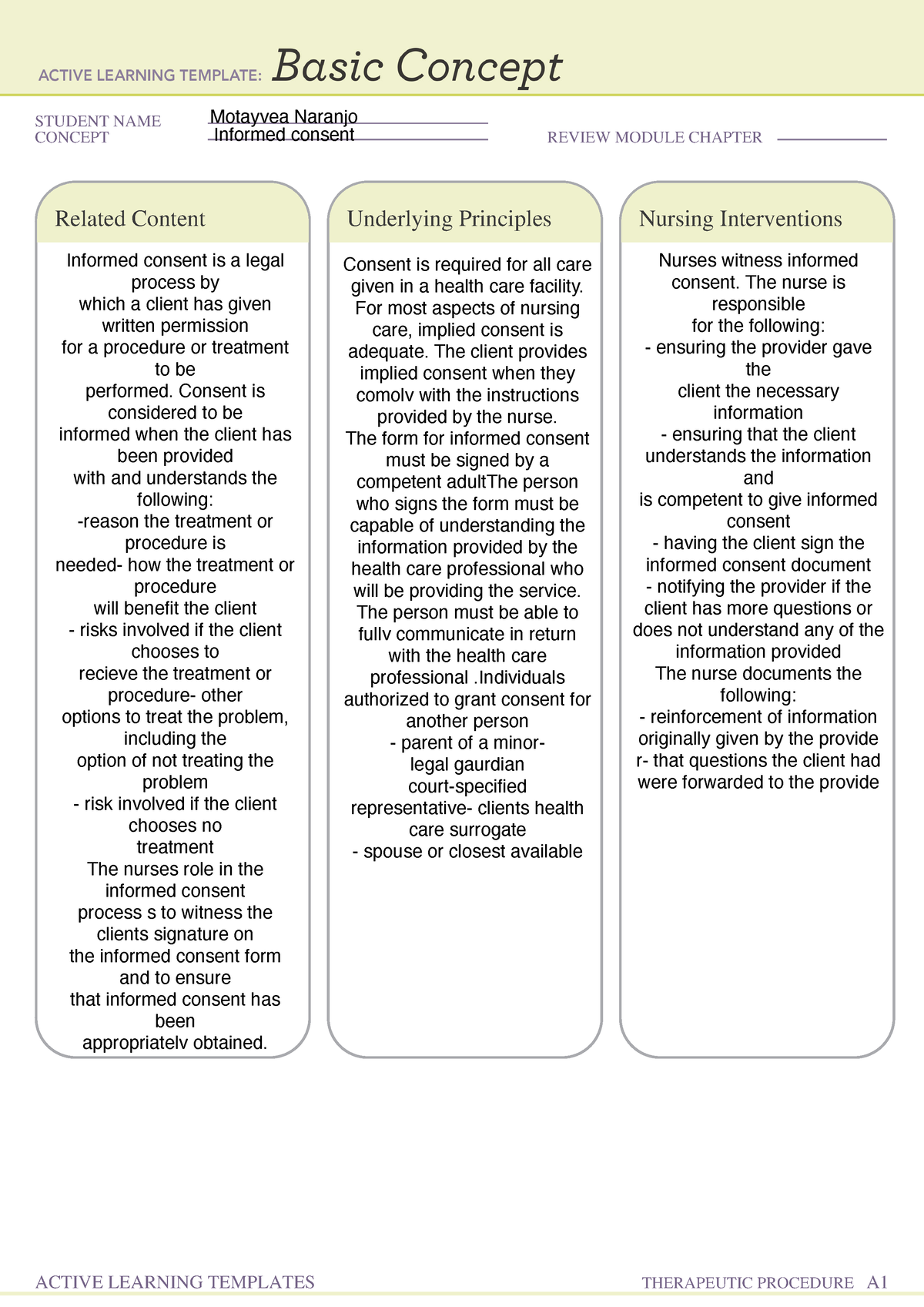
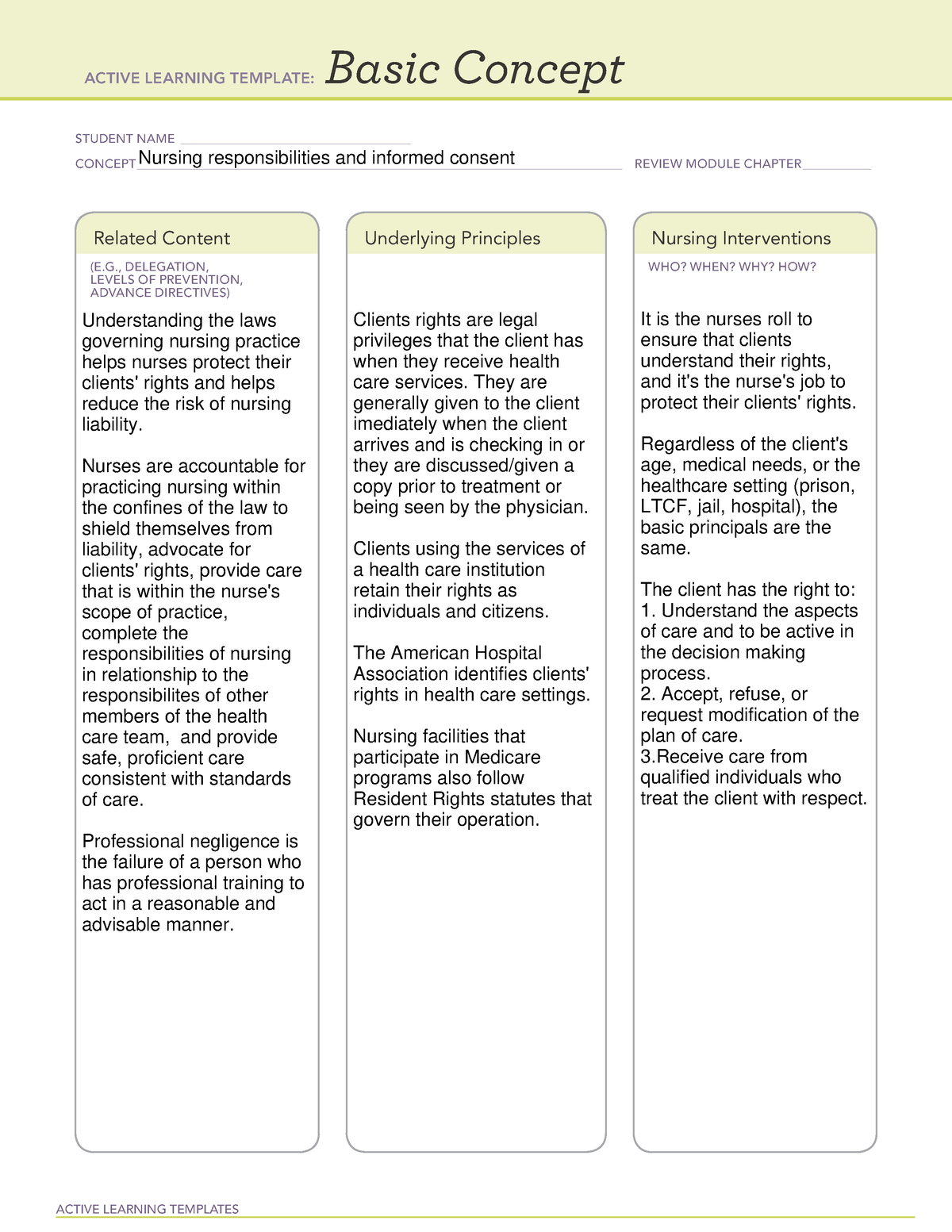
Webinformed consent must be organized and presented in a way that facilitates comprehension. When broad consent is obtained, the requirements imposed by 46. 116(a)(5) for the. Webunder the federal regulations, a pi must obtain “legally effective” informed consent in order to enroll a person into a research study. The “consent process” describes who will obtain. Weba general requirement for informed consent is that no informed consent may include any exculpatory language. Exculpatory language is that which waives or appears to waive. Send a copy of the informed consent via facsimile to the subject's wife. After she has had the opportunity to speak to the investigator, she can sign the informed consent and fax it. Weboct 4, 2024 · the purpose of informed consent is: To provide a potential subject with appropriate information in an appropriate manner and allow that person to make an. Weba general requirement for informed consent is that no informed consent may include any exculpatory language. Exculpatory language is that which waives or appears to waive. Webrecognize the elements of consent. Determine when waivers are appropriate. Identify methods for ensuring comprehension of consent. , distinguish between consent as a.
Informed Consent Citi Quizlet
Informed ConsentExample
Informed ConsentForm
Informed ConsentForm Printable
Informed Consentfor Therapy
Informed ConsentForm Template
Informed ConsentForm Counseling
Psychotherapy ConsentForm
Informed ConsentClip Art
MedicalInformed Consent
Informed ConsentLetter Sample
Patient Informed ConsentForm
Medical Negligence
Informed ConsentForm For Research